Abstract
Background. Marginal zone lymphomas (MZLs) are characterized by an indolent course with median survival of over 10 years. Unfortunately, relapses/progressions are common and might heavily impact patients' survival. Chimeric antigen receptor (CAR) T cells revolutionized the treatment of several relapsing/refractory B cell malignancies. However, CAR T cells success in MZL is still limited (Jacobson et al, Lancet Oncol 2022) and none of the currently available products has been approved so far for this indication. Little is known about the reasons behind the limited efficacy of CAR T cells against MZL.
Given the importance of CD19-antigen density in CAR T cell killing of B-cell malignancies, in this work we investigated the contribution of CD19 levels in sensitivity of MZL in vitro models to CAR T cells. We previously presented MZL-derived models with secondary resistance to BTK/PI3K inhibitors, also observing increased CD19 levels in some of the resistant cells (Arribas et al, ASH2019, ENA2019, Haematologica 2022). Based on these data, we hypothesized that the CD19 modulation induced by exposure to BTK/PI3K inhibitors might be a strategy to increase CAR T cell efficacy in MZL. Here, we tested this hypothesis using MZL models, parental or chronically exposed to BTK/PI3K inhibitors and with acquired resistance to the small molecules.
Methods. CD19 expression was determined at protein level by flow cytometry. CD19-specific CAR T with CD28 co-stimulatory domain were produced from healthy donors using retroviral vectors. Transduction efficacy was evaluated by protein L staining. MZL cells were co-cultured with the CAR T cells at an E:T ratio of 1:1 after adjusting for transduction efficacy. Viability was measured by flow cytometry at 48h. CAR T cell-mediated cytotoxicity was calculated as percentage of cell death compared to MZL cells cultured alone. Longitudinal cell growth was measured by bioluminescence in mCherry/Luciferase transduced cells after normalization to day 0.
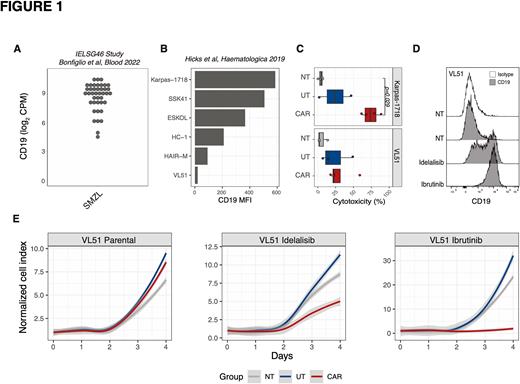
Results. As we previously reported, CD19 expression levels are very heterogenous both across MZL clinical specimens (n.=41; Bonfiglio et al, Blood 2022; Figure 1A) and cell lines (n.=6; Hicks et al, Haematologica 2019; Figure 1B). We selected models expressing the highest (Karpas-1718) and the lowest (VL51) CD19 levels and tested their sensitivity to CAR T cell killing. CD19-specific CAR T cells displayed a robust cytotoxic activity against Karpas-1718 (median 74%, range 61-88%) while no CAR T cell-specific killing was observed against VL51 (Figure 1C). The results were in agreement with the CD19 levels, higher in Karpas-1718 and lower in VL51. PI3K or BTK inhibition led to CD19 upregulation on VL51 surface (Figure 1D). Compared to the non-treated (NT), co-incubation of parental VL51 with CAR T cells or control untransduced T (UT) cells did not inhibit tumor cell growth (Figure 1E, left panel). Conversely, CAR T cells, but not control UT cells, led to 43% growth inhibition at day 4 after co-incubation with idelalisib-treated VL51 (Figure 1E, middle panel) and completely inhibited ibrutinib-treated VL51 growth (Figure 1E, right panel).
Conclusions. CD19 modulation by PI3K/BTK inhibition increased the sensitivity to CAR T cells in a CD19low MZL model. These data support exploring alternative modalities of CAR T cell treatment for MZL patients.
Disclosures
Simonetta:BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Travel support; AstraZeneca: Other: Travel support. Bertoni:ADC Therapeutics: Research Funding; Bayer: Research Funding; Helsinn: Research Funding; Menarini ricerche: Other: consulting fee, Research Funding; Neomed Therapeutics 1: Research Funding; Curis: Research Funding; Polyphor: Research Funding; Nordic Nanovector ASA: Research Funding; AstraZeneca: Other: travel support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal